Sunday Science: How To Supercharge Cancer-Fighting Cells – Give Them Stem-Cell Skills
Bioengineered immune cells have been shown to attack and even cure cancer, but they tend to get exhausted if the fight goes on for a long time. Now, two separate research teams have found a way to rejuvenate these cells: make them more like stem cells.
Both teams found that the bespoke immune cells called CAR T cells gain new vigour if engineered to have high levels of a particular protein. These boosted CAR T cells have gene activity similar to that of stem cells and a renewed ability to fend off cancer. Both papers were published today in Nature1,2.
The papers “open a new avenue for engineering therapeutic T cells for cancer patients”, says Tuoqi Wu, an immunologist at the University of Texas Southwestern in Dallas who was not involved in the research.
Reviving exhausted cells
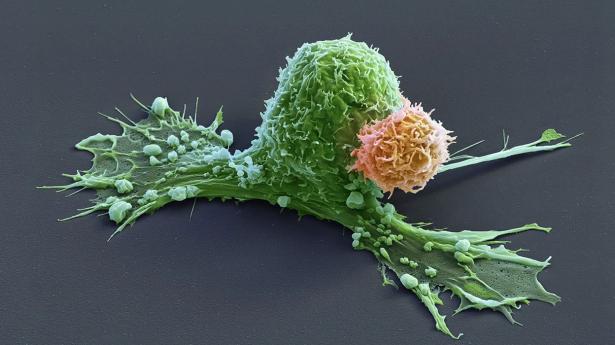
CAR T cells are made from the immune cells called T cells, which are isolated from the blood of person who is going to receive treatment for cancer or another disease. The cells are genetically modified to recognize and attack specific proteins — called chimeric antigen receptors (CARs) — on the surface of disease-causing cells and reinfused into the person being treated.
But keeping the cells active for long enough to eliminate cancer has proved challenging, especially in solid tumours such as those of the breast and lung. (CAR T cells have been more effective in treating leukaemia and other blood cancers.) So scientists are searching for better ways to help CAR T cells to multiply more quickly and last longer in the body.
With this goal in mind, a team led by immunologist Crystal Mackall at Stanford University in California and cell and gene therapy researcher Evan Weber at the University of Pennsylvania in Philadelphia compared samples of CAR T cells used to treat people with leukaemia1. In some of the recipients, the cancer had responded well to treatment; in others, it had not.
The researchers analysed the role of cellular proteins that regulate gene activity and serve as master switches in the T cells. They found a set of 41 genes that were more active in the CAR T cells associated with a good response to treatment than in cells associated with a poor response. All 41 genes seemed to be regulated by a master-switch protein called FOXO1.
The researchers then altered CAR T cells to make them produce more FOXO1 than usual. Gene activity in these cells began to look like that of T memory stem cells, which recognize cancer and respond to it quickly.
The researchers then injected the engineered cells into mice with various types of cancer. Extra FOXO1 made the CAR T cells better at reducing both solid tumours and blood cancers. The stem-cell-like cells shrank a mouse’s tumour more completely and lasted longer in the body than did standard CAR T cells.
Master-switch molecule
A separate team led by immunologists Phillip Darcy, Junyun Lai and Paul Beavis at Peter MacCallum Cancer Centre in Melbourne, Australia, reached the same conclusion with different methods2. Their team was examining the effect of IL-15, an immune-signalling molecule that is administered alongside CAR T cells in some clinical trials. IL-15 helps to switch T cells to a stem-like state, but the cells can get stuck there instead of maturing to fight cancer.
The team analysed gene activity in CAR T cells and found that IL-15 turned on genes associated with FOXO1. The researchers engineered CAR T cells to produce extra-high levels of FOXO1 and showed that they became more stem-like, but also reached maturity and fought cancer without becoming exhausted. “It’s the ideal situation,” Darcy says.
The team also found that extra-high levels of FOXO1 improved the CAR T cells’ metabolism, allowing them to last much longer when infused into mice. “We were surprised by the magnitude of the effect,” says Beavis.
Mackall says she was excited to see that FOXO1 worked the same way in mice and humans. “It means this is pretty fundamental,” she says.
Engineering CAR T cells that overexpress FOXO1 might be fairly simple to test in people with cancer, although Mackall says researchers will need to determine which people and types of cancer are most likely to respond well to rejuvenated cells. Darcy says that his team is already speaking to clinical researchers about testing FOXO1 in CAR T cells — trials that could start within two years.
And Weber points to an ongoing clinical trial in which people with leukaemia are receiving CAR T cells genetically engineered to produce unusually high levels of another master-switch protein called c-Jun, which also helps T cells avoid exhaustion. The trial’s results have not been released yet, but Mackall says she suspects the same system could be applied to FOXO1 and that overexpressing both proteins might make the cells even more powerful.
doi: https://doi.org/10.1038/d41586-024-01043-2
References
-
Doan, A. et al. Nature https://doi.org/10.1038/s41586-024-07300-8 (2024).
-
Chan, J. D. et al. Nature https://doi.org/10.1038/s41586-024-07242-1 (2024).
Sara Reardon: I’m a freelance journalist covering biomedical, environmental and social science from beautiful Bozeman, Montana. I've previously worked as a staff reporter at Nature, New Scientist and Science. In my previous life, I studied mouse sperm and retinas. Realizing that I could never limit my interests to one field of science, I switched to science journalism -- a field that thrills me every day. I've reported from five continents and all over the United States. My favorite stories involve the myriad ways in which science intersects with society, particularly through law, ethics, and culture. I also make short films about science and health-related issues affecting my local community. Follow me on Twitter to see my latest work and drop me a message if you have ideas or tips!
Nature is a weekly international journal publishing the finest peer-reviewed research in all fields of science and technology on the basis of its originality, importance, interdisciplinary interest, timeliness, accessibility, elegance and surprising conclusions. Nature also provides rapid, authoritative, insightful and arresting news and interpretation of topical and coming trends affecting science, scientists and the wider public.
Nature's mission statement
First, to serve scientists through prompt publication of significant advances in any branch of science, and to provide a forum for the reporting and discussion of news and issues concerning science. Second, to ensure that the results of science are rapidly disseminated to the public throughout the world, in a fashion that conveys their significance for knowledge, culture and daily life.
Nature's original mission statement was published for the first time on 11 November 1869.
About the Editors
Like the other Nature titles, Nature has no external editorial board. Instead, all editorial decisions are made by a team of full-time professional editors. Information about the scientific background of the editors may be found here.

New Discoveries Demystify the Bizarre Force That Binds Atomic Nuclei Together
Stanley J. Brodsky, Alexandre Deur, Craig D. Roberts
Scientific American
April 12, 2024

Spread the word